Capturing sunlight with supercomputing
Summary
Converting sunlight into electricity is now commonplace, with efficient silicon-based solar cells steadily becoming more affordable. But manufacturing them from very pure silicon will always be a relatively slow and energy-intensive process. Alternatives to silicon solar cells are in development which promise to be just as efficient, simpler to manufacture, and cheaper – if they could be produced at commercial scale with improved stability. Dr Asaph Widmer-Cooper from the University of Sydney node of the ARC Centre of Excellence in Exciton Science is studying how these materials are created and break down at the atomic level, to guide efforts to make them commercially viable.
Image above: Roll to Roll Perovskite. Courtesy of CSIRO.
The Challenge
Metal halide perovskites are a class of minerals that could revolutionise renewable energy generation. Perovskite solar cells can capture sunlight as efficiently as silicon, but can be manufactured in a completely different way. Rather than melting, slowly crystallising and then slicing fine wafers from a large crystal, as is done with silicon to make solar cells and computer chips, metal halide perovskites can be formed into thin crystalline films by depositing a solution of their component parts onto an appropriate base and then removing the solvent, effectively ‘printing’ the solar cell on a surface.
A continuous printing process offers a low-cost route to solar cells on a range of surfaces, but while these can be produced at small scale, it is very difficult to form uniform thin films of perovskite at large scale. Trial-and-error printing is narrowing down the conditions needed to reproducibly produce thin crystalline layers, but very small changes in crystallisation conditions can lead to big differences in final product performance. Researchers still need a deeper understanding of how perovskite crystals form at the molecular level, and how to influence their crystallisation to make a more robust and reproducible commercial product. They also need a better understanding of how these thin films degrade in the presence of water, and how the insertion of other molecules into the crystalline film can inhibit that process.
Complementing practical printing experiments, Dr Widmer-Cooper is using computational modelling to study the crystallisation and dissolution of perovskites at the molecular level. The atomic structure of the crystal and its light-capturing properties are well known, but modelling its formation from solution requires calculating the behaviour of millions of atoms for long enough to observe crystal nucleation and growth. “The model is just a set of mathematical rules governing how the various ions and molecules in both the solution and at the surface interact with each other,” explains Dr Widmer-Cooper, “but there’s a trade-off between the accuracy of the model we can use and the amount of time we can run it for”.
The Solution
Using Pawsey supercomputing, Dr Widmer-Cooper has tested a range of models to develop one that describes perovskites accurately both in the crystalline state and in solution.
“To see crystal growth we have to run the model for thousands of nanoseconds, recalculating atomic movements each quadrillionth of a second,” says Dr Widmer-Cooper. “We can only do that using a very large CPU cluster like Magnus, or a GPU cluster like Topaz.”
Dr Widmer-Cooper and his team are now using their model to ‘watch’ how the crystals grow from solution and dissolve. “The next step is to explore those mechanisms, using the model to test how they are affected by variations in temperature, concentration, solvent and additives.”
“Using this model, we’re hoping to provide some molecular-level insight into the variations that occur in large-scale perovskite printing processes, and suggest approaches to test to make thin-layer crystallisation more reproducible and robust for commercial production.”
Outcome
Once we really understand the mechanisms of crystallisation and dissolution and what affects them, we can create the most stable conditions to scale up perovskite printing for commercial production. It’s a process worth pursuing, as Dr Widmer-Cooper has already seen what can be achieved at an experimental scale.
“The first commercial outcome is likely to be printing perovskite on top of existing silicon solar cells. Perovskites can be tuned to absorb different parts of the solar spectrum than and silicon, so a dual-layer cell will absorb and convert more sunlight to electricity than a silicon solar cell alone.”
From there, the aim will be to create fully printable solar cells, with the potential to create solar panels on a range of different surfaces – even curved or flexible ones. All at a fraction of the effort and cost of using silicon.
Project Leader.
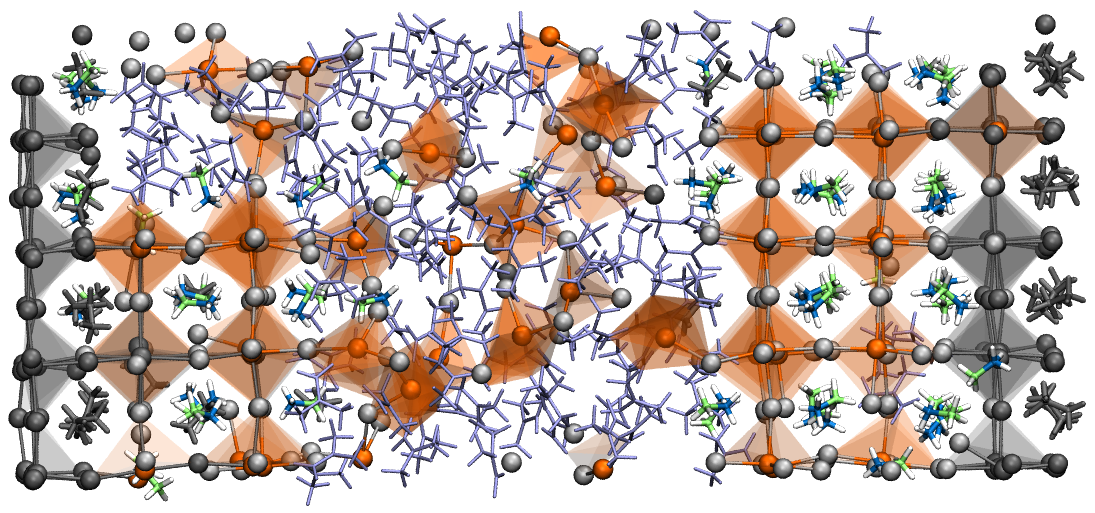
Simulation snapshot of a growing perovskite crystal. Image courtesy Asaph Widmer-Cooper
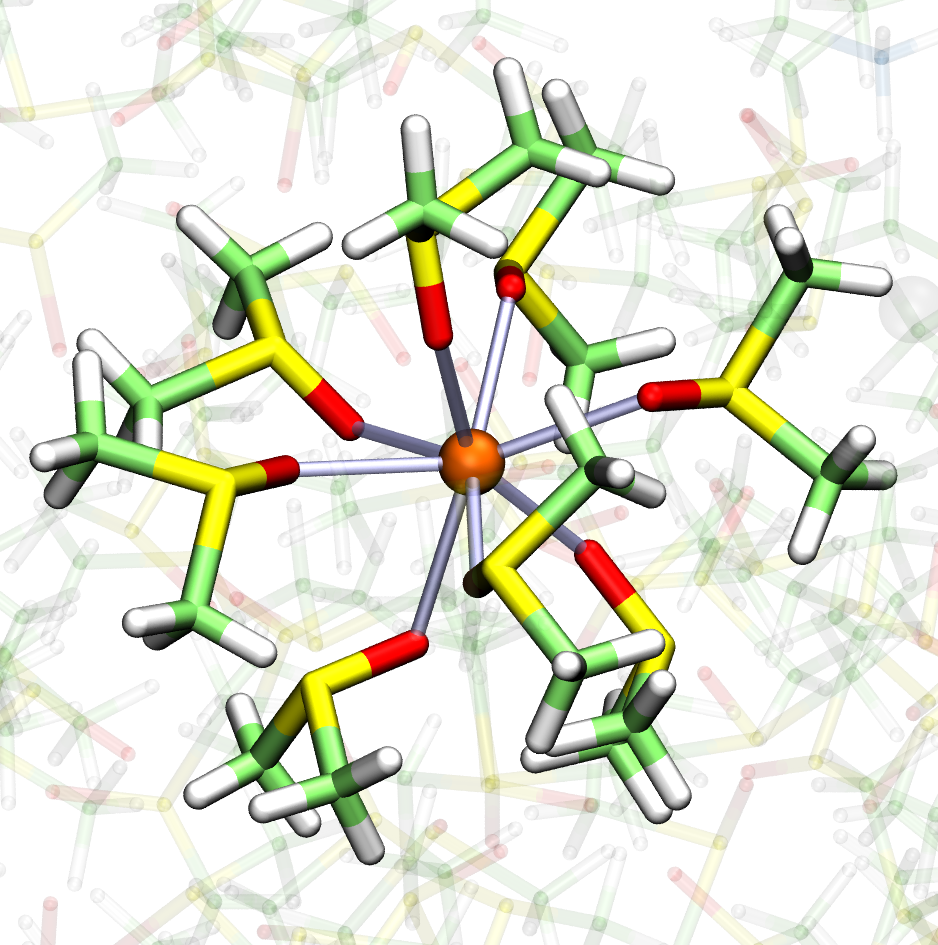
Simulation snapshots of perovskite precursors in solution. Image courtesy Asaph Widmer-Cooper